Maria-Luiza Prioteasa’s newborn baby, Sarah, was less than ten days old when she first worried something was wrong. She’d lost weight and had constant nappy rash.
At six weeks old, Sarah still wasn’t putting on weight. Then she developed a lung infection that antibiotics couldn’t clear.
Baffled, a midwife referred baby Sarah to hospital for more checks – including blood tests and X-rays – and the results were alarming.
Maria-Luiza, 32, a hospital support worker from south Yorkshire, was told her baby might have an immune deficiency.
‘I didn’t really know what it meant, but I found out that unless treated early, it could be fatal, which was terrifying,’ says Maria-Luiza.
Blood tests confirmed that Sarah had ADA severe combined immunodeficiency (ADA-SCID), a rare genetic condition affecting babies, that stops their immune system developing. ‘It felt like a physical blow to my heart,’ says Maria-Luiza. ‘I burst into tears.’
ADA-SCID affects around three children a year, and leaves them vulnerable to everyday germs the rest of us take in our stride.
Even minor infections, such as a cold, can be life-threatening. Without treatment, children with ADA-SCID usually die before their second birthday.
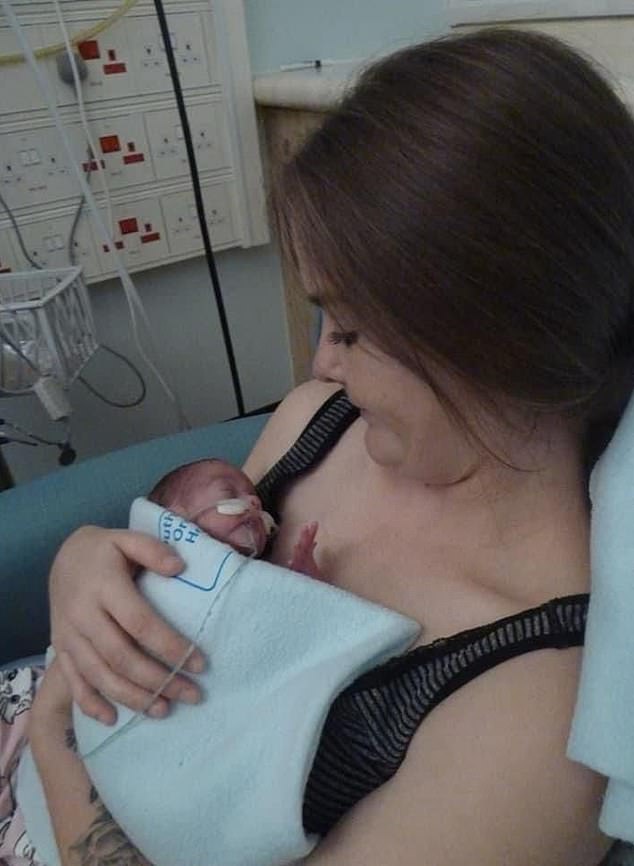

Yet today, Sarah is a healthy and happy eight-year-old who enjoys karate, swimming and ballet – something that would have been unthinkable without a pioneering gene therapy she had as part of a clinical trial.
The idea of putting babies like Sarah, even desperately sick ones, through a clinical trial might sound brutal to an outsider or an anxious new parent.
But such trials represent a lifeline when other options have failed, says Claire Booth, a paediatrician and a professor in gene therapy and paediatric immunology at the UCL Great Ormond Street Institute of Child Health, in London.
‘It gives them a future when certain illnesses mean they won’t survive otherwise.’
What’s more, the only way to improve treatment for all babies is if medication and care options are studied in babies.
In Sarah’s case, her condition was caused by a faulty gene, meaning she couldn’t produce the enzyme, ADA, which helps the immune system develop. It meant taking every precaution to keep her safe from infections that might kill her.
‘This meant mopping the floors several times a day, cleaning door handles, washing my hands after touching my face and before touching her,’ says Maria-Luiza. Even kissing her or sleeping close to her at night was too much of a risk. ‘Not being able to kiss my own baby was torture,’ she adds.
The best option, Sarah’s family were told, would be an invasive bone marrow transplant, which could replace the unhealthy immune system with new, healthy blood-forming stem cells to build a new immune system.
But first Sarah would need to be matched with a stem-cell donor before going through chemotherapy to kill off her own existing unhealthy cells.
Unfortunately, a match could not be found, and Sarah was referred to Great Ormond Street Hospital (GOSH) in London to discuss other treatments, including weekly injections of PEG-ADA, a replacement for Sarah’s missing ADA enzymes. But the injections were expensive – around £8,000 for every 2ml vial – and doctors weren’t confident it would be a success.
Then a third option was raised: taking part in a clinical trial.
Sarah seemed to be a good fit for a GOSH trial for a new kind of gene therapy, where the faulty gene is replaced with a working copy. Doctors use a virus to carry the working gene into the patient. There was no guarantee it would work – but, if successful, immune cells would start to develop.
Maria-Luiza admits she didn’t let herself fear the worst: ‘I needed to be there for her. It wasn’t until a couple of years later that I fully realised how scary it was.’
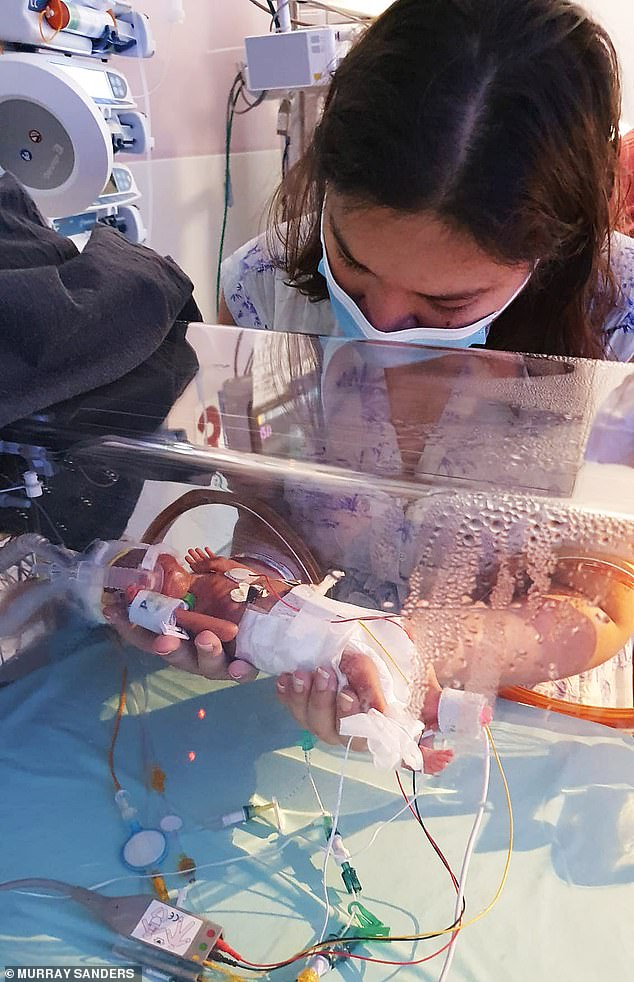
At the time Sarah joined the trial, the GOSH team had successfully treated a number of other patients with the one-off therapy.
‘The procedure itself is not dangerous,’ says Professor Booth.
‘It works by collecting the patients’ blood stem cells using a machine very similar to a kidney dialysis machine. We then work in the lab to produce a working copy of the same gene, before giving it to the patient.’ Maria-Luiza had many questions, but ultimately felt she had ‘no choice’ but to give it a try. ‘As a parent, you will do everything you can to save your child,’ she says.
Sarah had her treatment at six months old. Blood tests showed immune cells had begun to grow within weeks.
‘All her symptoms, like rashes, started to improve.’ says Maria-Luiza. ‘She was smiling and happier, with clearer skin and brighter eyes. It was amazing to see.’
From there, Sarah made a full recovery. ‘I’ve no doubt that if Sarah hadn’t had access to this treatment, she would not be alive today,’ says Maria-Luiza, who’s since begun a nursing degree and works supporting parents of children admitted to GOSH.
In the past, medicines prescribed for children were generally only tested on and formulated for adults due to reluctance to conduct studies on children for ethical reasons.
However, there’s been a shift in opinion since the mid-1990s, and a lack of data on the effect of drugs on babies is now seen as a concern.
‘Children and babies aren’t just mini adults,’ says Professor Booth. ‘You can’t necessarily extrapolate from adult trials what would happen in children because they have a different body chemistry and so can react to treatments differently.’
This is partly due to their size but also, because their bodies are still developing, the effects on infant organs may be different, she explains. ‘It’s really important to tailor treatments for them – and the way we do that is by doing research and clinical trials.’

Researchers are keen to enrol babies with more common conditions on to trials so experts can collect data and learn more about their unique physiology.
The fact is that studies involving real babies are crucial because laboratory testing cannot replace real patients, explains Neena Modi, a professor of neonatal medicine at Imperial College London.
‘If you want to bring about benefits for babies then you have to involve babies,’ she says.
But baby trials bring their own challenges. For one thing, babies are especially vulnerable and require specialist monitoring equipment and care in specialist centres and hospitals with appropriate equipment and trained staff.
And parents may be understandably anxious – especially if their baby is ill or premature and they have to make a quick decision about whether to enrol them.
‘Parents are inevitably and understandably in a heightened state of worry and concern about their baby,’ says Caroline Lee-Davey, chief executive of Bliss, a charity for premature and sick babies.
‘We always want to see informed consent – meaning the parents understand what the trial is aiming to do and exactly how it might impact their child,’ she says. ‘Ideally they’ve had time to process the request and think about it before agreeing to take part.’
There may be benefits for parents in choosing to have their baby take part in a trial, adds Professor Modi.
A 2020 study, involving parents of children who’d taken part in neonatal drugs trials in Belgium, found that overall they reported ‘positive experiences and little emotional distress after participation’. Almost all reported satisfaction and pride in contributing to research, while a minority reported anxiety, stress or guilt.
Any study involving babies must pass strict ethical tests, as adult trials do, before being approved, explains Professor Modi.
At each stage, the researchers face scrutiny by relevant regulatory authorities. ‘So by the time a parent of a baby in an intensive care unit is presented with the study, that study will have been scrutinised heavily by a number of different bodies,’ says Professor Modi.
And very often studies in babies do not involve new drugs or invasive treatments, says Caroline Lee-Davey.
‘In neonatal care it’s often about measuring the difference between one standard practice and another,’ she says. This might involve, for instance, the timing of surgery after birth or studying how often a baby is being fed – ‘things that are less black and white’.

One recent study involved asking parents to stroke their babies during the standard heel-prick test, typically done at five days old to check for conditions such as sickle cell disease and cystic fibrosis. The researchers sought to measure whether parental touch could reduce discomfort and distress felt by babies during the test.
Katie Stanmore’s son Benjamin took part in a 2019 trial after he was born prematurely, at 26 weeks.
Scans shortly after he was born revealed he had an extra blood vessel in his heart, a condition known as patent ductus arteriosus (PDA).
PDA affects around one in every 2,000 full-term babies, but the risk is much higher for children born before 29 weeks.
For most babies with otherwise normal hearts, the extra blood vessel will shrink and close on its own within the first few days of life.
If it stays open for longer, however, it can cause extra blood to flow to the lungs, damaging the smaller blood vessels and risking permanent damage, which can be fatal.
‘It was a big shock when Benjamin came so early, I was overwhelmed with emotion and worry,’ says Katie, 31, from Southport. During her pregnancy, Katie suffered from hyperemesis gravidarum – severe nausea and vomiting – which meant she was ‘in and out of hospital, and bleeding from around 16 weeks of pregnancy… it was such an awful time,’ she recalls. Benjamin was born via emergency Caesarean at Liverpool Women’s Hospital weighing just 1lb 7oz (652g).
‘When I went to see him in the neo-natal intensive care unit he was covered in wires,’ says Katie. ‘His skin was see-through and he was making kitten-like noises.’
When Benjamin was around two days old, a consultant told Katie about a clinical trial at the University of Oxford.
Researchers wanted to test whether administering ibuprofen to newborns with PDA could help to close up the extra blood vessel when administered in the first 72 hours of a baby’s life.
There were no guarantees it would work, however, and none of the families taking part would be told whether their babies were given the drug or the placebo.
‘I said yes straight away,’ says Katie. ‘Of course it was a worry, but I reassured myself that the experts knew what they were doing.’
A scan of Benjamin’s heart after the drug was given via intravenous drip showed his valve had successfully closed. ‘The staff were absolutely amazing and talked me through everything,’ says Katie.
Benjamin was in hospital for 76 days in total and, due to lung development issues, he required oxygen at home until he was six months old.
But now, aged four, he is ‘fit, well and very happy,’ says Katie. ‘You’d never think he was a premature baby by looking at him. He was a little fighter from day one.’
While the level of regulation and safety precautions involved means ‘it should be exceptionally rare that the research could be actively harmful to a baby’, Caroline Lee-Davey says it’s possible for parents to be left feeling disappointed if they do not get the outcome they had hoped for – for example, if their baby is given the placebo treatment over a new intervention.
‘I can understand that disappointment for parents if they think they haven’t benefited from the new thing that maybe would have helped their baby,’ she says. ‘But that is what research is about: it’s often comparing something new against the existing standard practice. It’s not the case that one baby gets better care than the other.’
Katie won’t ever know if Benjamin got the ibuprofen or a placebo. ‘It’s always been on my mind,’ she says, ‘but all I can do is be grateful for the trial regardless.’
In studies involving both healthy and sick babies, ethics are always a consideration, says Professor Booth. ‘For the gene therapy trials our team work on, we do not complete comparison studies [with a placebo] as it would be unethical to do this,’ she explains. ‘And in rare diseases, it may not be possible to have a placebo arm of the trial due to the limited number of patients, for example.’
Though he didn’t directly benefit, Mia Roberts-Mukasa is glad she let her son Oscar take part in research into necrotizing enterocolitis (NEC), a gastrointestinal condition that occurs mainly in premature babies. Gut tissue becomes inflamed and dies off, leading to life-threatening infection.
Oscar was born at 27 weeks, weighing just 1lb 10oz (737g). ‘It was a very scary time for us, we didn’t know if he would survive,’ says Mia, 31, an events producer from Hampshire.
In the days after Oscar’s birth at St Thomas’ Hospital in London in October 2021, Mia and her husband were approached about research trials taking place on the neonatal unit. ‘One was to test the use of an antibiotic as a prophylactic [i.e. to prevent infection] as opposed to treatment for infection. But I didn’t like the idea of giving him antibiotics if there was no medical need.
‘We decided to opt into the NEC trial, which seemed the least invasive and potentially most relevant to him,’ says Mia.
Babies who contract NEC usually undergo an emergency operation to remove part of the intestine. If Oscar did contract NEC, the family consented to let surgeons take an extra piece of his intestine during the procedure, for further study.
Thankfully, Oscar wasn’t affected, but he stayed on the trial as a ‘control’, being monitored as a comparison against babies who did develop NEC. ‘It was a positive experience and we didn’t feel under any pressure,’ says Mia.
‘We also felt that, God forbid, he might not make it, at least we’d be making some lasting impact that might help other babies.
‘Knowing you’re contributing to something bigger that might help others can really give meaning to an awful time.’
Only a few days old… and helping scientists unravel the secrets of our brains
Just a few days old, wrapped in swaddling and its head covered in electrodes, this newborn baby undergoes a test to measure the electrical activity of its brain.
But this is no emergency intervention to try to save the life of a critically ill infant. In fact, it’s an experiment to see if newborns have an intrinsic understanding of the rhythm of music.
The test, carried out by scientists at the University of Amsterdam in the Netherlands, involved performing an electroencephalogram (EEG) – where electrodes are used to monitor brain activity that indicates its response to external changes.
In this study, 31 babies – all under six days old – wore earphones, through which researchers played a drum beat of the kind heard in most pop songs.

When a single beat was dropped from the pattern, the EEG registered a change in the babies’ brain activity – indicating they were able to sense a shift in the drum rhythm, even though they’d never been exposed to these noises before.
Writing in the journal Cognition earlier this year, the researchers said the findings showed babies are born with a sense of rhythm that’s fundamental to humans’ appreciation of music.
But is it ethical to perform experiments on tiny babies – especially experiments where there is no obvious medical benefit, such as this study?
This question is covered by the Declaration of Helsinki, a complex set of ethical principles first drawn up by the World Medical Association in 1964, to set the boundaries for who should and should not be human guinea pigs for research.
These state that ethics committees – experts who vet all medical research projects – must decide whether participating in experiments amounts to ‘more than minimal risk’ for the participant.
‘Anything that an ethics committee perceives as more than minimal risk is very unlikely to be allowed to go ahead,’ says Dominic Wilkinson, a professor of medical ethics at the University of Oxford.
‘In this case, exposing babies to drum beats would be judged to be fine if the committee was happy that it didn’t mean being exposed to any more noise than they would be in everyday life,’ he says.
‘But if the researchers planned to put headphones on a baby and play it heavy metal music very loudly that would not be allowed.’
In the drum-beat experiment, noise levels were capped at around 80 decibels – roughly the same as a vacuum cleaner.
Pat Hagan

